
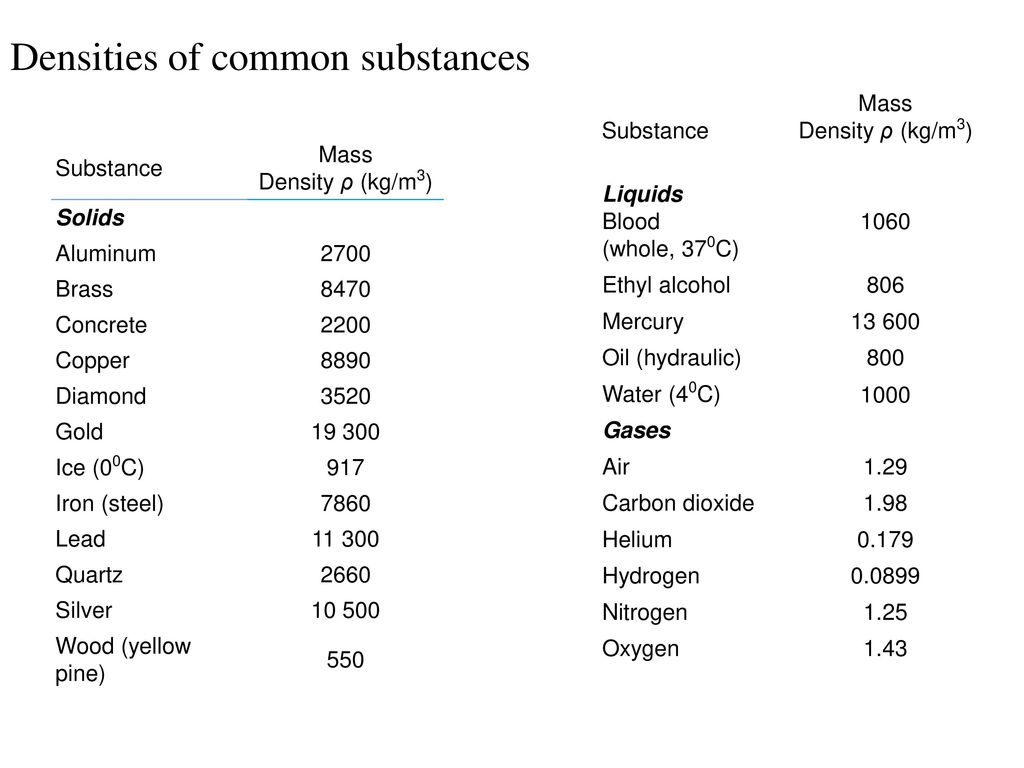
Ice at 0 C. Water at 4 C.
Water for example has a density of 10 gcm 3 grams per cubic centimeteranother way to say this is gmL which is grams per milliliter.
Densities of common liquids. 249 rijen Densities of common liquids like acetone beer oil water and more. Engineering ToolBox -. 262 rijen Oxygen liquid-183-2974.
31 rijen liquid. Calculate the density of each liquid by dividing its mass by its volume 50 ml. Record the density of each liquid on Data Table 1.
After filling each cell click the diskette icon in the bottom menu bar to continue. Click the table icon in the bottom menu bar to access Data Table 2. It shows the densities of some common liquids.
Density of common fluids. Density gcm3 at 20C and 1 atm unless noted water 099820 water 0 C 099984 water 4 C 099997 water 100 C 095836 gasoline 066-069 ethyl alcohol 0791 turpentine 085 olive oil 09 castor oil 0969 sea water. 103 milk 1028-1035 glycerin 1260 mercury.
Densities of common liquids. November 14 2020 By. Do you need to convert any type of pressure unit.
This means that the value of density is independent of the quantity of matter present. Use this data to calculate the density of the metal in gcm. Water at 4 C.
Water at 20 C. Ice at 0 C. Density gmcm3 Gases at STP.
Liquids - Densities - Densities of common liquids like acetone beer oil water and more. Weight - Mass vs. Weight - the Gravity Force.
Metals and Alloys - Densities - Density of some common metals metallic elements and alloys - aluminum bronze copper iron and more. Mineral Densities - Densities of minerals. Page 2 of 4 CCWCCUSD 100213 ActivityLesson WARMUP Ashleyhasthreeliquidsthatshewantstopourintoajarwateroilandhoney Herfriendsallhad.
Densities of common minerals1 Earth Science abilities to do inquiryGrades 412Distributed by Women in Mining Education Foundation Revised 2007 DENSITIES OF COMMON MINERALS STANDARDS See summary of National Science Education Concept General Standard Specific Standard General Standard Specific Standard General Standard Specific Standard Grade Science as Inquiry A. Every liquid has a density number associated with it. Water for example has a density of 10 gcm 3 grams per cubic centimeteranother way to say this is gmL which is grams per milliliter.
Here are the densities of the liquids used in the column as well as other common liquids. Specific gravity of common liquids and fluids like alcohol oils benzene water and many more Specific gravity of a liquid is the ratio of the density of the liquid - to the density of water at 4oC. Specific gravity of some common liquids and fluids.
T oC 59 T oF - 32. Ever especially in the case of liquids to express Density is the mass per unit volume and is all specific gravities in terms of water at 40 C. Usually expressed in grams per cubic centimeter The values are then directly comparable since or per milliliter at some definite temperature.
Weigh each liquid in grams make sure you subtract the weight of the beaker and then divide that number by the volume number of milliliters of the liquid. The answer is density in grams per milliliter. Your answer will be more exact if you use a graduated cylinder instead of a beaker to measure the volume and weigh the liquid.
Liquid densities are largely independent of pressure but they are somewhat temperature-sensitive. The density range of solids is quite wide. Metals whose atoms pack together quite compactly have the highest densities although that of lithium the highest metallic element is quite low.
The density of pure water is also 624 lbscuft pounds per cubic foot and if we know that a sample of ethyl alcohol has a sg of 0785 then we can calculate that its density is 0785 x 624 49 lbscuft. Note kgcum divided by 1602 lbscuft. 3 liquids of different densities choose from.
Honey corn syrup dish soap water with a drop of food colouring vegetable oil food colouring. Which liquid is densest. How can you tell.
Show students the three liquids and ask them to predict which liquid has the highest density and which liquid has the lowest density.