However sweating is not an exothermic reaction. These energy changes are either occurring naturally combustionburning photosynthesis respiration boiling freezing etc or being employed by mankind to make our lives better through its numerous application.
Here are some examples from everyday life.
Endothermic reaction in everyday life. Endothermic reactions are those reactions that require absorbing energy to happen. The reactions that release energy when they happen are known as Exothermic reactions. Since energy absorption is required in endothermic reactions many of the compounds formed in these reactions are unstable and any loss of energy causes them to disappear.
There is a single feature which may be used to identify any endothermic reaction and that is the absorption of heat. Here are some examples from everyday life. The whole food pyramid on Earth is sustained by the presence of photosynthesizing plants that.
Endothermic Chemical Reactions The reaction of barium hydroxide octahydrate crystals with dry ammonium chloride Dissolving ammonium chloride in water The reaction of thionyl chloride SOCl 2 with cobalt II sulfate heptahydrate Mixing water and ammonium nitrate Mixing water with potassium chloride. Endothermic Process vs Endothermic Reaction. The human body exploits the endothermic nature of evaporation to cool itself.
This is done through the process of sweating. The sweat produced on the surface of the skin absorbs heat from the skin to evaporate thereby creating a cooling effect. However sweating is not an exothermic reaction.
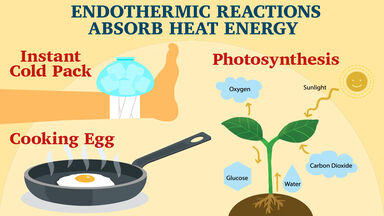
An endothermic reaction is a process or reaction in which the system absorbs energy from its surroundings. Usually but not always in the form of heat. Photosynthesis is an endothermic reaction where light energy is required.
The light is absorbed so that the plant can produce sugars and oxygen. This is the most common type of endothermic reaction in everyday life. Switching on an electric light bulb.
Applications of exothermic and endothermic reactions in everyday life Application of exothermic and endothermic reactions. The principle of exothermic and endothermic reactions is applied in instant cold packs and hot packs which are used to treat sports injuries. Moreover how are endothermic reactions used in everyday life.
The process of cooking food is endothermic as you have to give the food energy to have a chemical reaction. In this reaction energy in is absorbed from the sun in order to take carbon dioxide and water and using the solar energy convert it to glucose and. Endothermic Reaction Defined Chemical reactions are all about the energy.
In an endothermic reaction heat is used for the reaction to occur. The heat energy breaks the bonds in the substance causing the reaction. For example if theres a cup of hot chocolate on a table with the system being the cup the reaction is exothermic because heat is leaving the cup and going to the table but the reaction is endothermic for the table because it is receiving the heat from the cup.
Endothermic reactions occur when new molecules being made form more new bonds than those that are broken. The net result of these new bonds being made is that energy is absorbed from the surroundings to make these new bonds and the reaction has a decrease in temperature or gets cooler. Endo- means inside and therm means heat.
Endothermic and Exothermic Reactions Processes are commonly observed in our everyday life. These energy changes are either occurring naturally combustionburning photosynthesis respiration boiling freezing etc or being employed by mankind to make our lives better through its numerous application. One of the most common application is the.
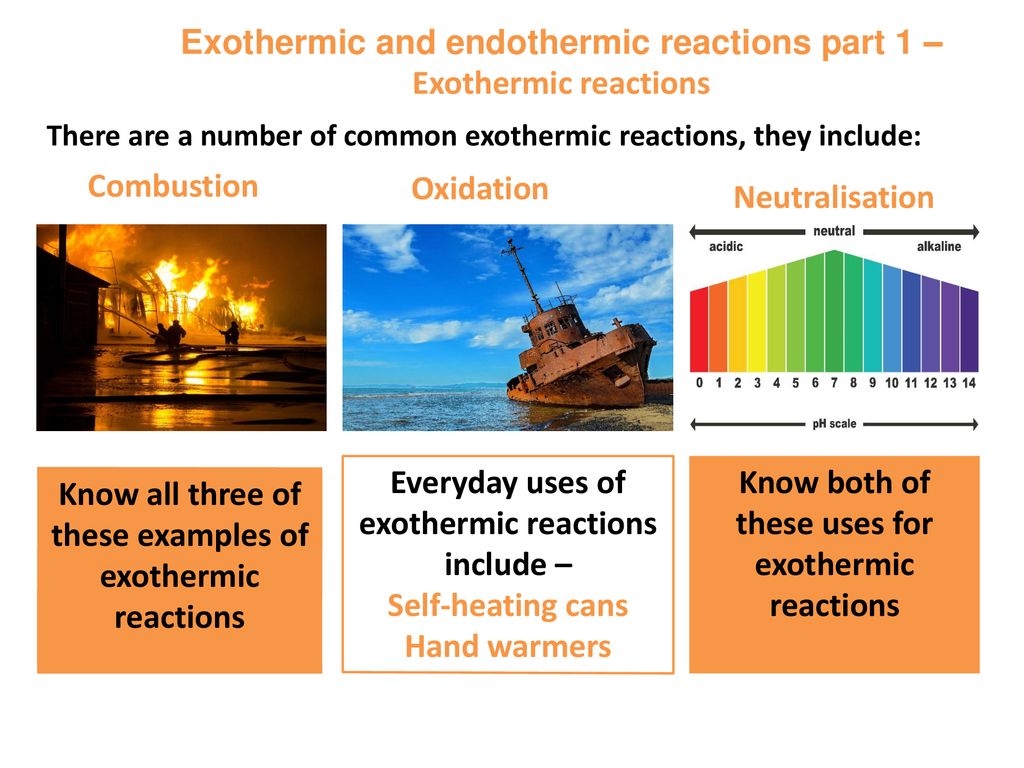
Everyday uses of endothermic reactions include instant ice packs which can be used to treat sports injuries. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid and an endothermic reaction between sodium. 8 Examples of Exothermic Reaction in Everyday Life 1.
Cellular Respiration This is an essential exothermic reaction that occurs in every cell and provides energy to our. Ice Cubes When water freezes into ice cubes the energy is released in the form of. Plants apply a chemical reaction called photosynthesis to convert carbon dioxide and water into food glucose and oxygen.
Its one of the most common everyday chemical reactions and also one of the most important because this is how plants produce food for themselves and animals and convert carbon dioxide into oxygen. Endothermic and Exothermic Reactions Processes are commonly observed in our everyday life. These energy changes are either occurring naturally combustionburning photosynthesis respiration boiling freezing etc or being employed by mankind to make our lives better through its numerous application.
An endothermic reaction is a type of chemical reaction in which energy is consumed in the form of heat so that the product obtained has an energy greater than the initial reagents. The term endothermic has Greek roots. Endo which means inside and thermos hot which would mean absorbing heat.